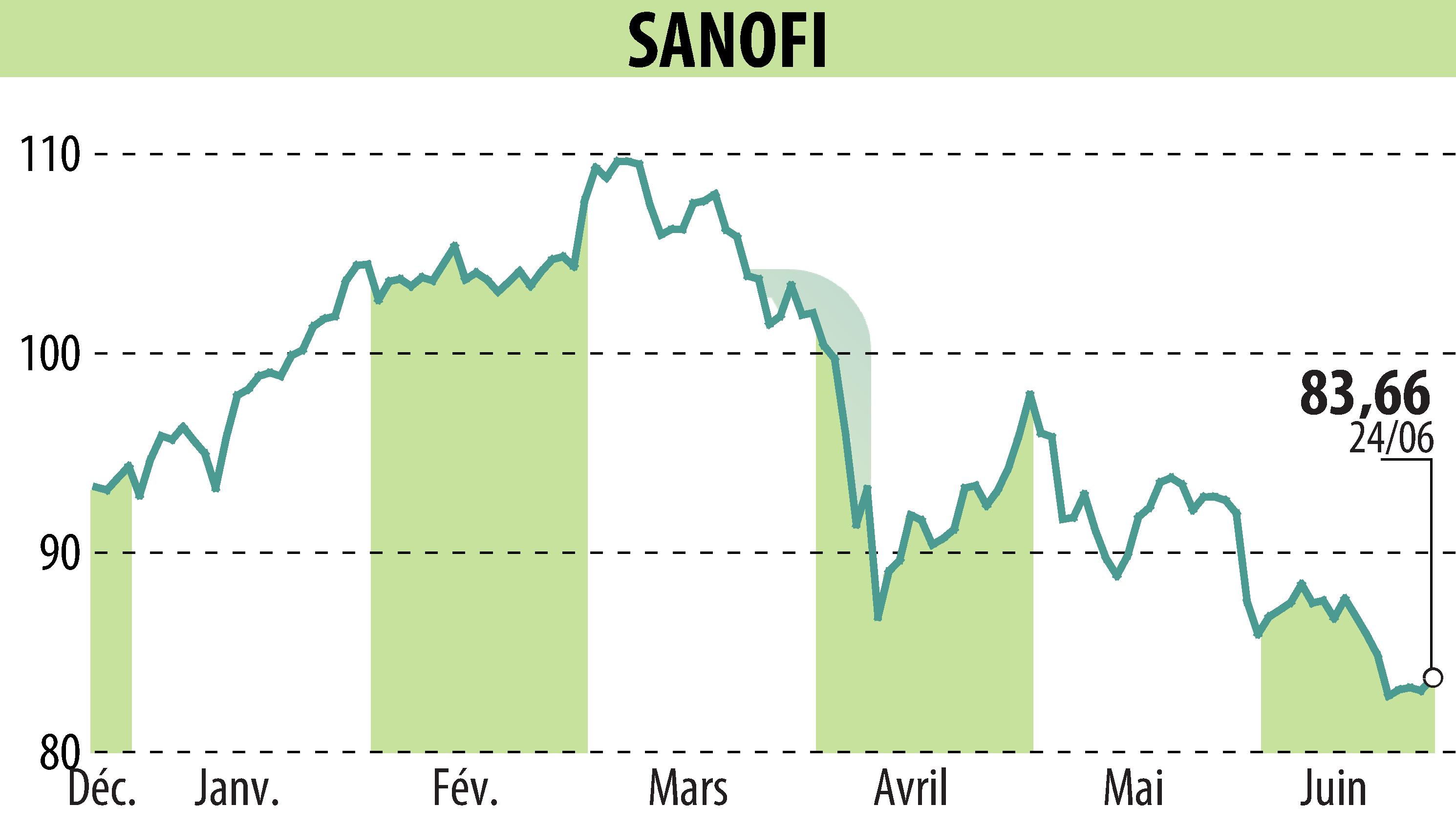
on SANOFI-AVENTIS (EPA:SAN)
Riliprubart Granted Orphan Drug Designation in the United States
The U.S. Food and Drug Administration (FDA) has designated riliprubart as an orphan drug to treat antibody-mediated rejection in solid organ transplants. Sanofi is pursuing a Phase 2 study to evaluate the potential of this treatment in kidney transplant recipients. Riliprubart addresses an unmet medical need, as there are currently no FDA-approved treatments for this condition. This investigational treatment could represent a significant advancement for patients at risk of rejection. In parallel, Sanofi is exploring its effectiveness in other rare diseases such as chronic inflammatory demyelinating polyneuropathy.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
