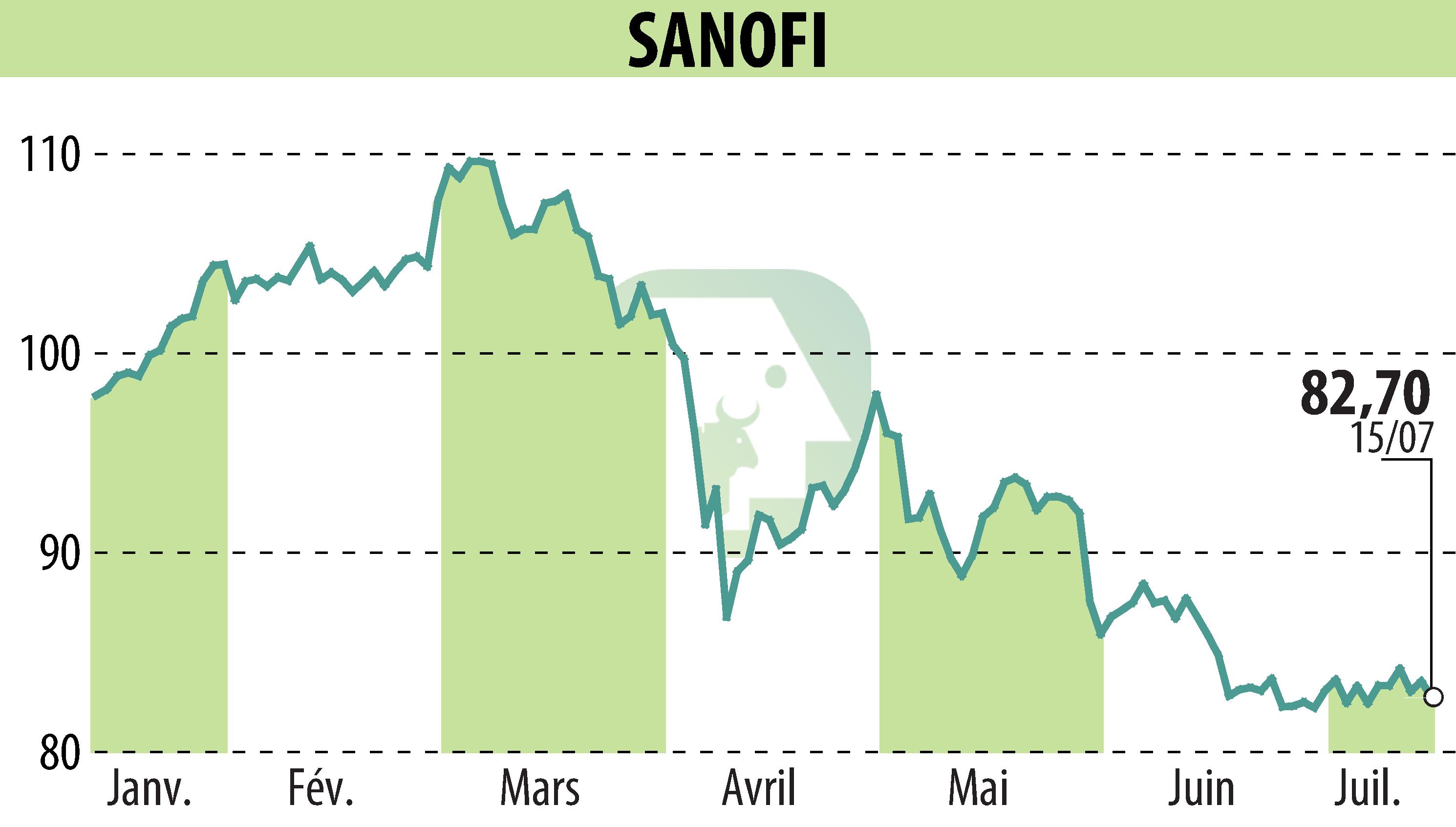
on SANOFI-AVENTIS (EPA:SAN)
Sanofi: Fast track designation for SAR446597 in the United States
Sanofi has received fast-track designation from the U.S. FDA for SAR446597. This treatment targets geographic atrophy due to age-related macular degeneration. SAR446597 is a single-dose intravitreal gene therapy. It inhibits two key pathways in the complement cascade, reducing the need for frequent injections.
Sanofi's strategy aims to accelerate the development of treatments for serious diseases. A Phase 1/2 study will evaluate SAR446597 for safety and efficacy. Sanofi is continuing its research with SAR402663 for other forms of macular degeneration.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
