on SANOFI-AVENTIS (EPA:SAN)
Sanofi's two combination vaccines against influenza and COVID-19 receive expedited review
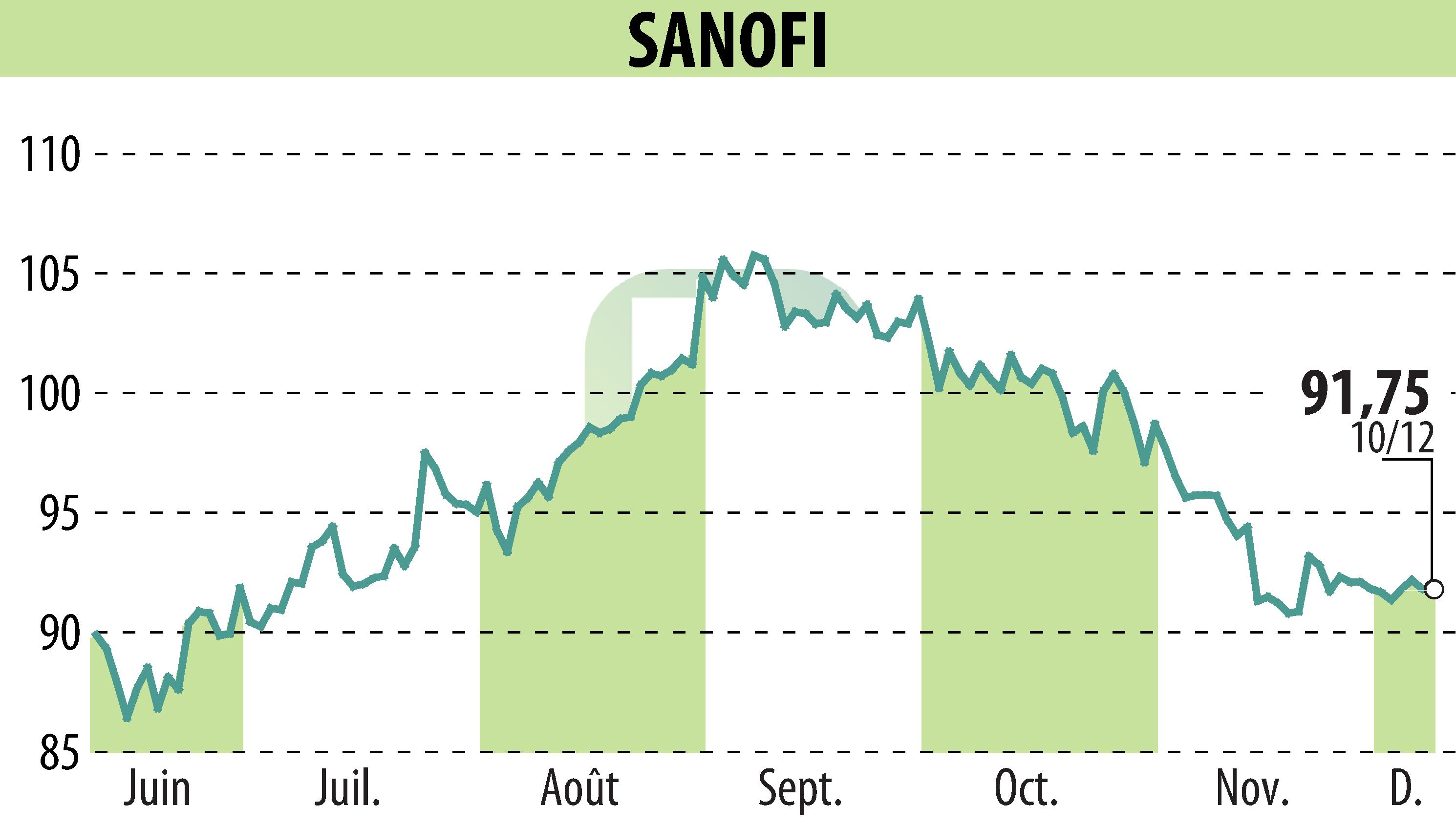
Sanofi has received accelerated review from the FDA for two combination vaccine candidates to prevent influenza and COVID-19 infections. Intended for adults 50 years of age and older, the vaccines combine formulations that are already approved and have proven efficacy. The first vaccine combines Fluzone High-Dose with the Novavax COVID-19 vaccine, while the second combines Flublok with the same vaccine. Phase I/II clinical studies are underway to evaluate their safety and immune response.
Combination vaccines aim to simplify vaccination by reducing the number of injections needed and decreasing the risk of hospitalization. A meta-analysis suggests that these vaccines would increase adult acceptance of boosters.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
