on SANOFI-AVENTIS (EPA:SAN)
Sarclisa Approved in the United States for the Treatment of Multiple Myeloma
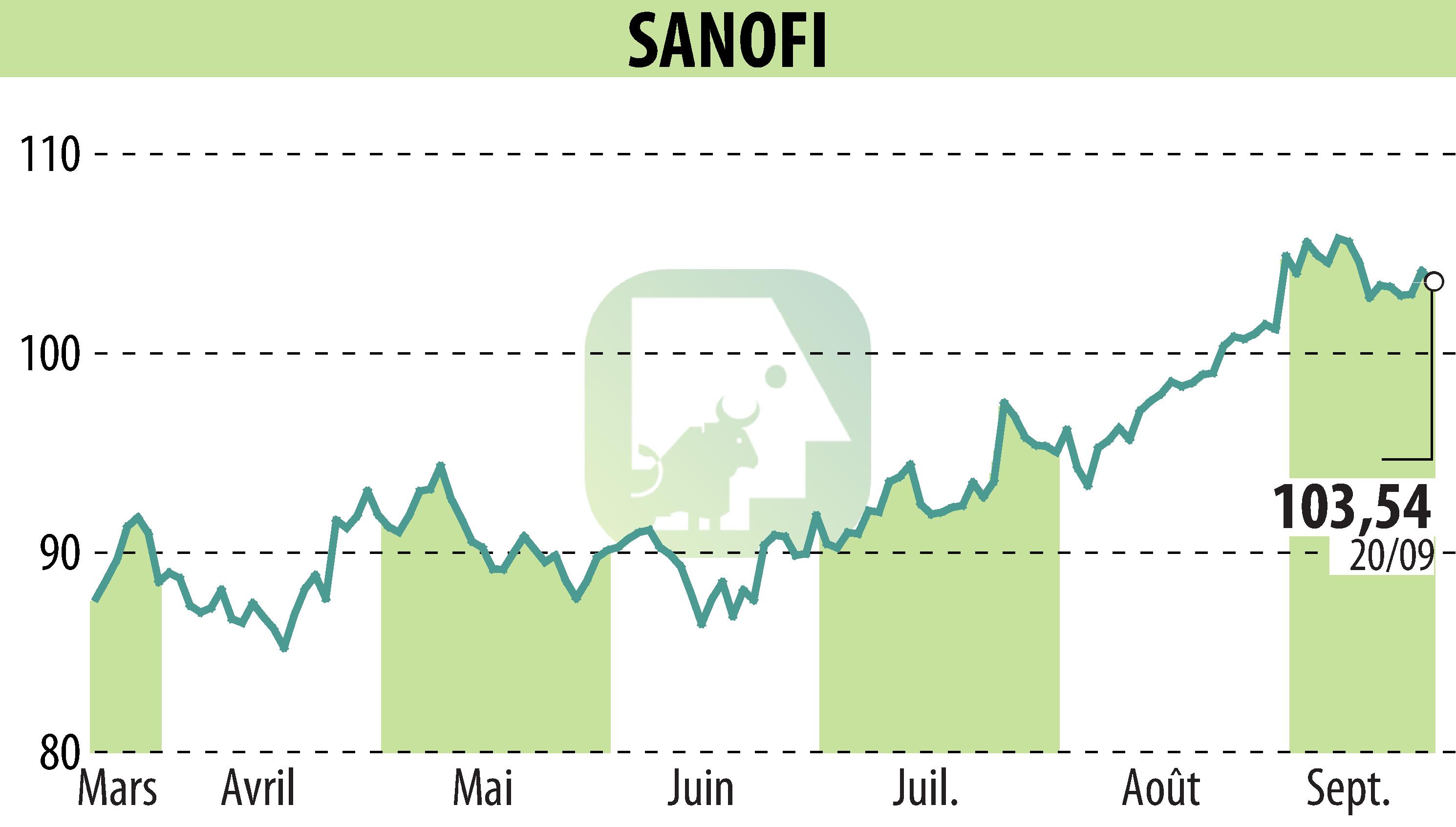
Sanofi-Aventis announced that the U.S. Food and Drug Administration (FDA) has approved Sarclisa (isatuximab) in combination with bortezomib, lenalidomide and dexamethasone (VRd) for patients with newly diagnosed multiple myeloma (NDMM) who are ineligible for stem cell transplantation. This decision is based on results from the Phase 3 IMROZ study, which showed a significant improvement in progression-free survival compared to VRd treatment alone.
Professor Thomas Martin, a specialist at the University of California, says the approval provides a much-needed new treatment option for older patients. Brian Foard, a vice president at Sanofi, says the approval provides a potentially life-changing drug for this serious disease.
Sarclisa is now approved for three indications in the United States, reinforcing Sanofi’s commitment to meeting the needs of patients with multiple myeloma. The treatment is already approved in more than 50 countries for the management of relapsed or refractory multiple myeloma.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
