on SENSORION (EPA:ALSEN)
Sensorion announces its 2025 half-year results
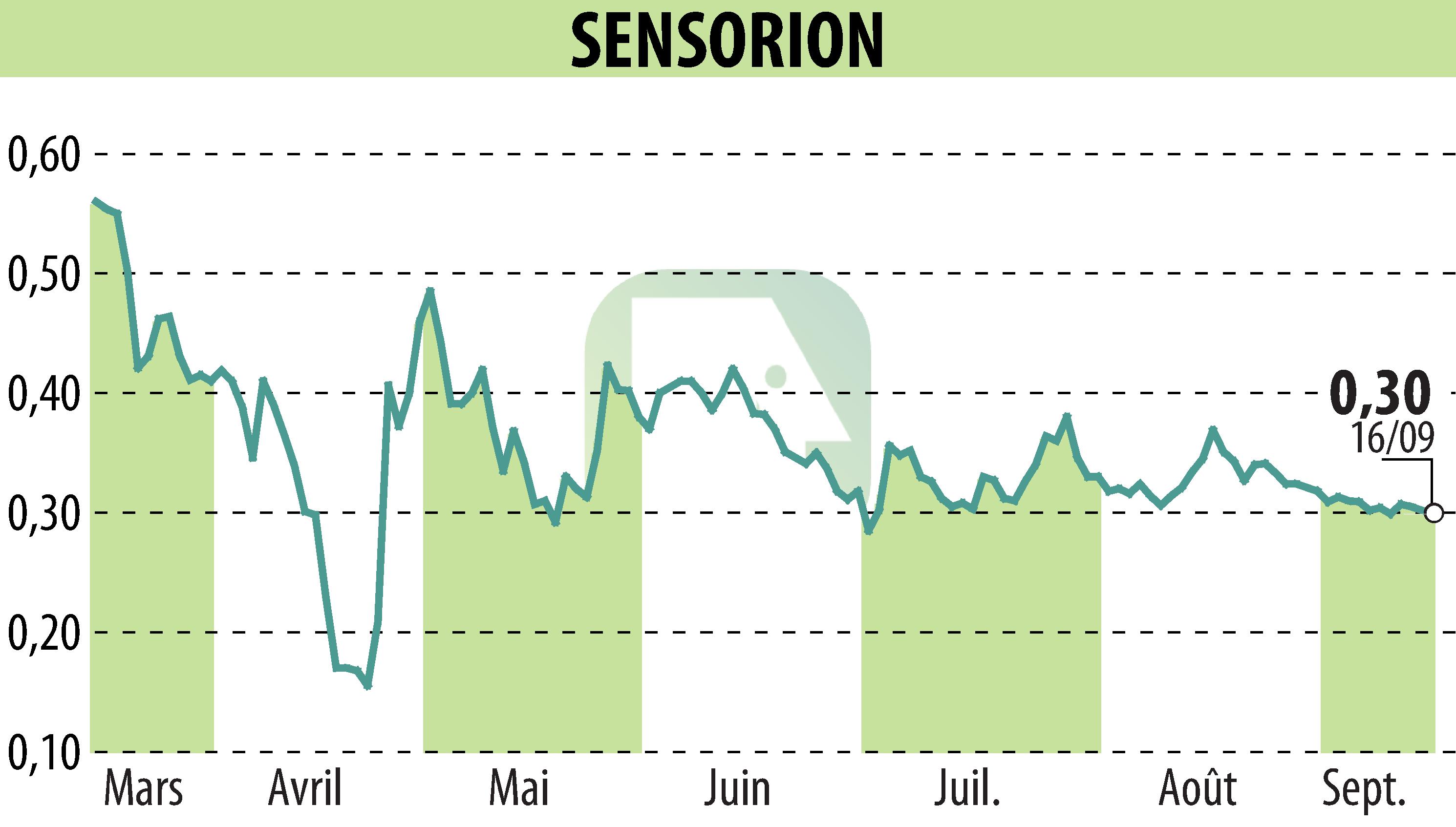
Sensorion, a biotechnology company specializing in the treatment of hearing disorders, has presented its results for the first half of 2025. Among its flagship projects, SENS-501, a gene therapy drug candidate, showed promising signs of hearing improvement in a patient. This Phase 1/2 study aims to treat congenital deafness caused by mutations in the OTOF gene.
In particular, Sensorion is preparing to file a clinical trial application for the GJB2-GT program in Q1 2026. The SENS-401 program, targeting cisplatin-induced ototoxicity, is continuing its progress with results expected by the end of 2025.
Financially, the company has €57.1 million in cash, ensuring the financing of its activities until the third quarter of 2026. Research and development expenses increased slightly to €15 million, while the net loss amounted to €16 million.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SENSORION news
