on SENSORION (EPA:ALSEN)
Sensorion's SENS-501 Clinical Trial Progresses with Positive DMC Recommendation
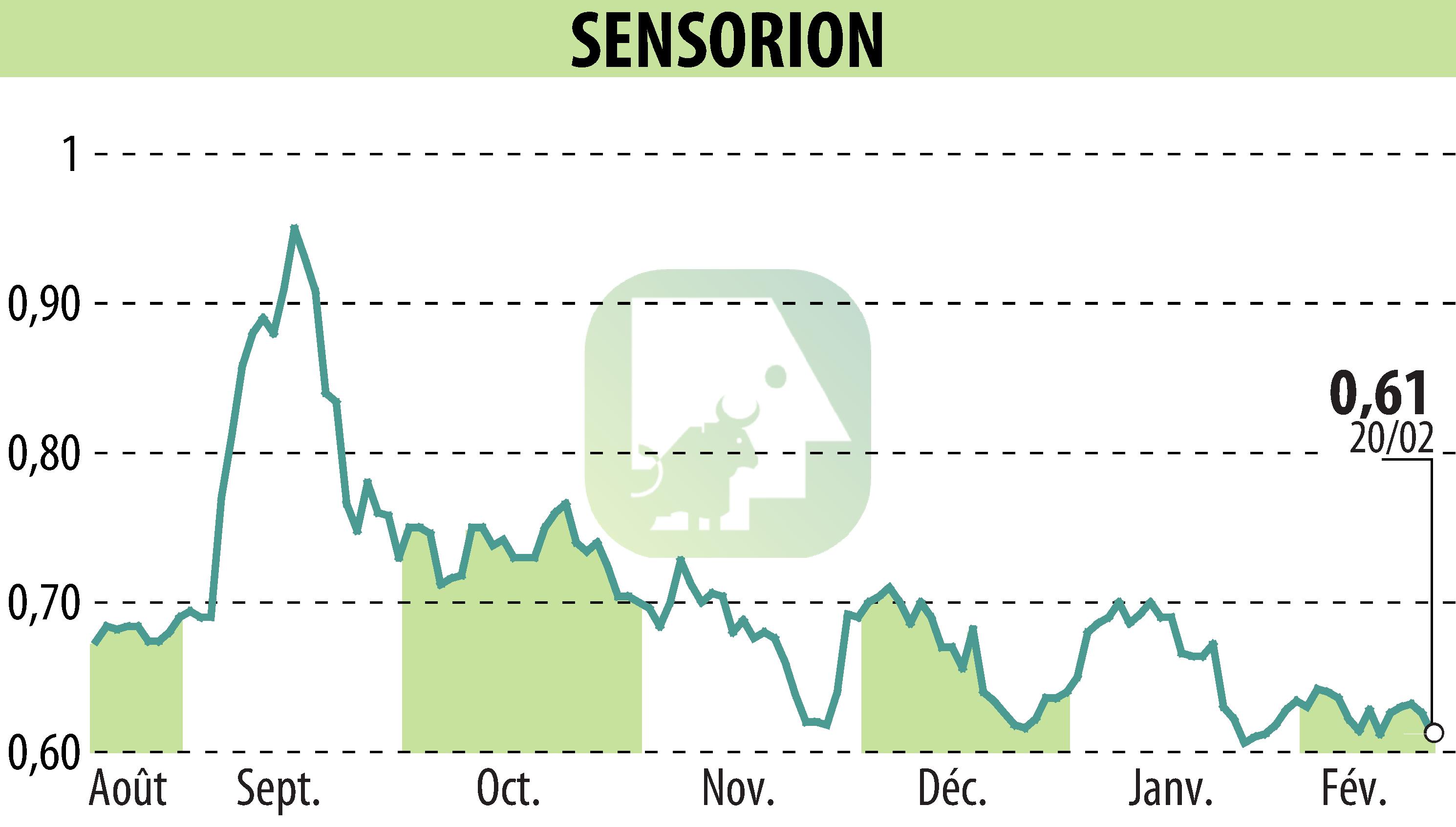
Sensorion, a clinical-stage biotech company focused on hearing loss therapies, announced a positive recommendation from the Data Monitoring Committee (DMC) for its Audiogene Phase 1/2 trial of SENS-501. This gene therapy targets congenital deafness related to OTOF gene mutations. The DMC's review affirmed the safety of the first dose level in young children, leading to the trial's continuation without changes.
The first cohort's recruitment of three patients, children aged 6 to 31 months, was completed in December 2024. Recruitment for the second cohort is on track to conclude by mid-2025. The Audiogene trial aims to determine the safety and efficacy of SENS-501 through intra-cochlear injections, potentially improving speech and language acquisition in children with pre-linguistic hearing loss.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SENSORION news
