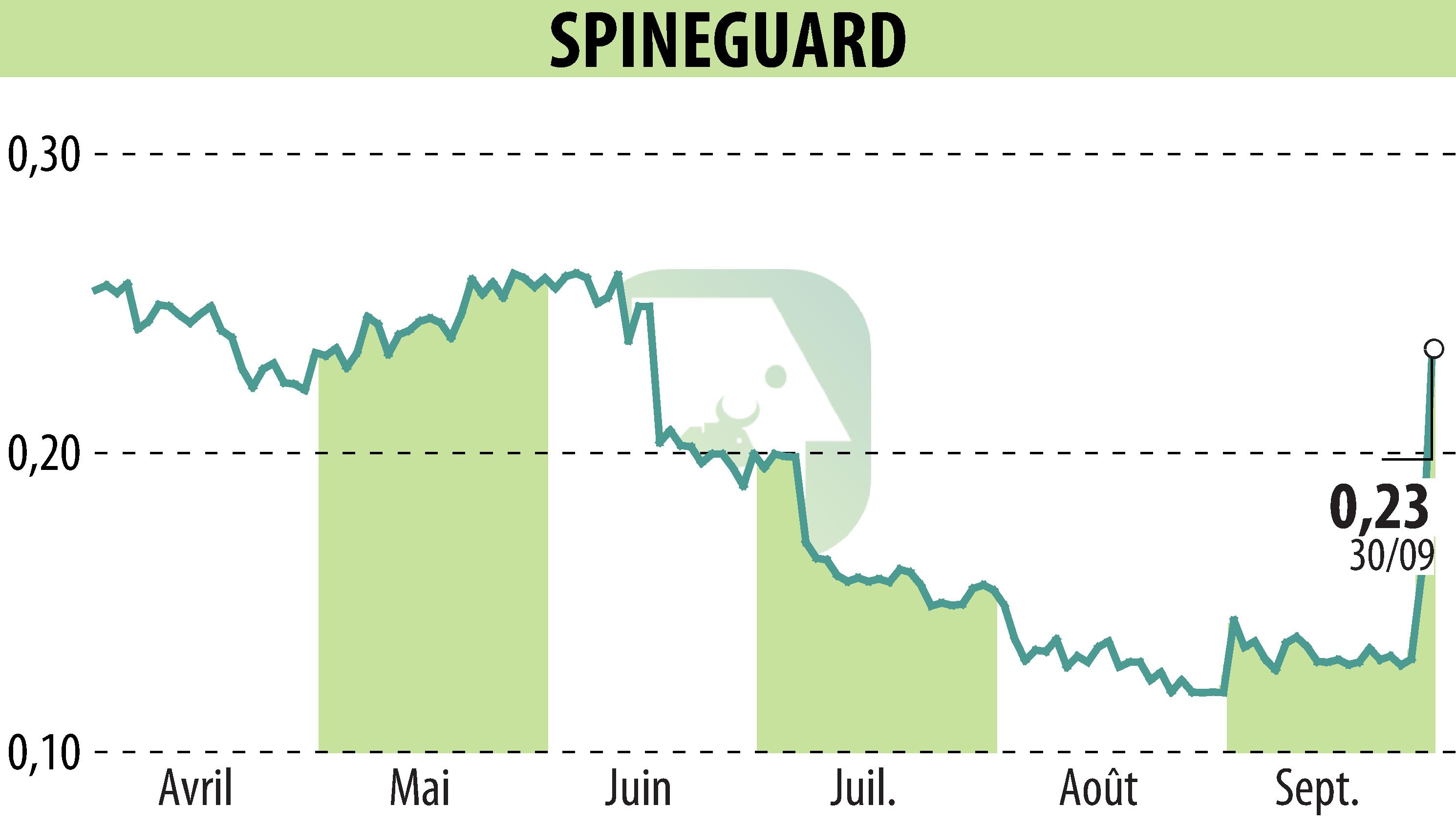
on SPINEGUARD (EPA:ALSGD)
SpineGuard Secures FDA Clearance for New PsiFGuard Device
SpineGuard has announced it received FDA clearance to commercially release its new smart drilling device, PsiFGuard, in the United States. Developed with US-based Omnia Medical, PsiFGuard is designed to secure Posterior sacroiliac Fusion (PsiF) procedures. This marks a significant milestone for the deployment of SpineGuard's DSG® technology in the US market.
Stéphane Bette, Deputy CEO and Co-Founder of SpineGuard, emphasized the efficacy of the PsiFGuard device in pre-clinical testing for locating the SI joint. He highlighted the broadening scope of their DSG technology and the exciting collaboration with Omnia Medical.
Troy Schifano, CEO and Co-Founder of Omnia Medical, echoed these sentiments, noting the importance of precise implant placement for successful SI joint fusion. He praised PsiFGuard's ability to enhance accuracy, thereby increasing the chances of surgical success.
The global sacroiliac fusion market was valued at $539 million in 2021 and is expected to grow substantially. A new payment code in the US for sacroiliac joint fusion surgery is likely to accelerate market growth.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SPINEGUARD news
