on POXEL (EPA:POXEL)
Sumitomo Pharma and Poxel Unveil Study Results of TWYMEEG® for Type 2 Diabetes
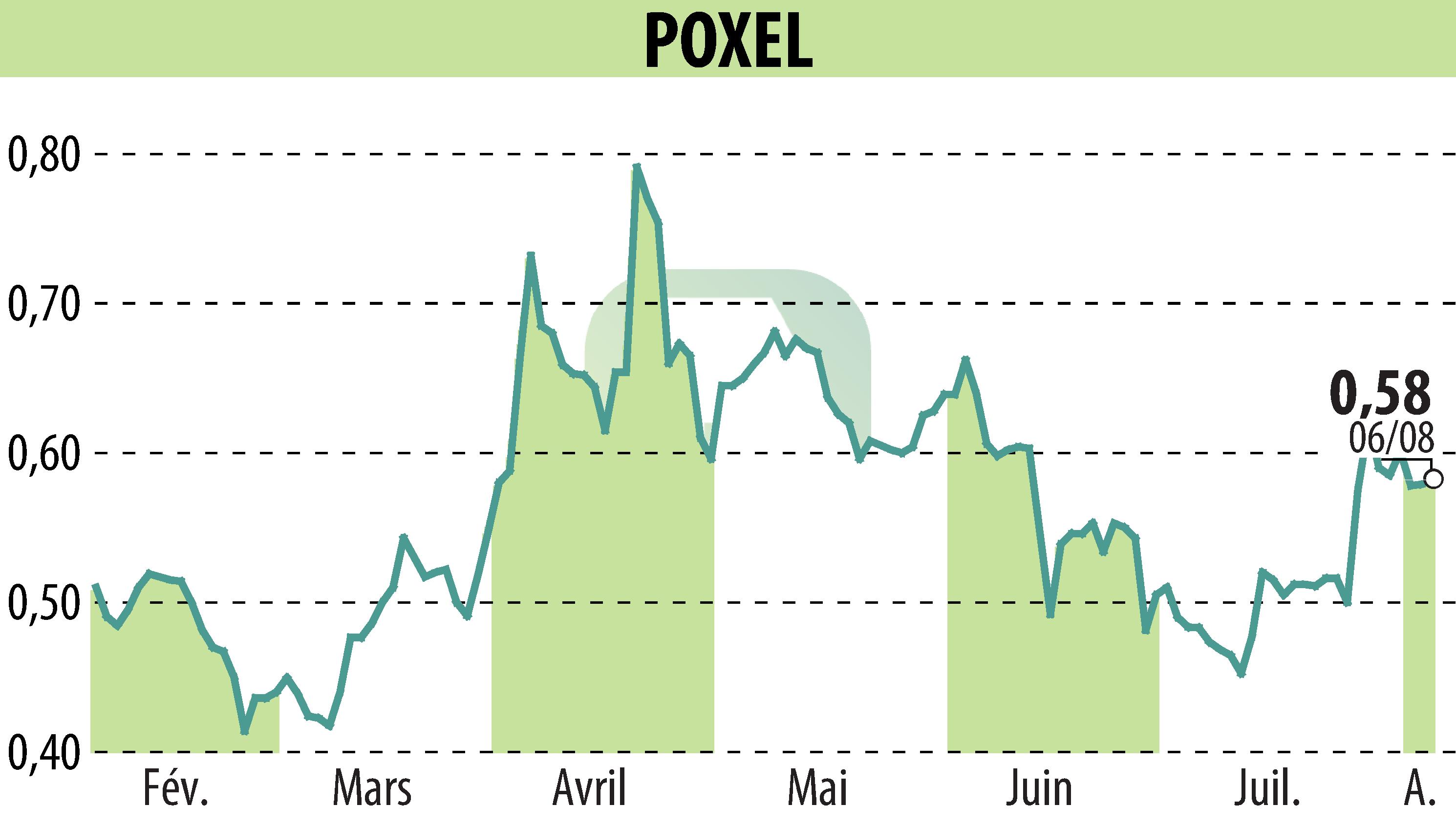
Sumitomo Pharma and Poxel have published preliminary results from the TWINKLE study, investigating TWYMEEG® for Japanese patients with type 2 diabetes and renal insufficiency. The study reveals a safety and tolerability profile consistent with previous studies.
Carried out open-label without a control group, this 52-week study involved 60 Japanese patients. The drug, administered in doses of 500 mg, showed serious adverse effects in 16.7% of cases but without a causal link.
These results prompt Sumitomo Pharma to discuss with Japanese authorities to potentially revise the drug's package insert for patients with an eGFR below 45 ml/min/1.73m² by fiscal year 2024.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all POXEL news
