on Moderna, Inc. (NASDAQ:MRNA)
Taiwan FDA Approves Moderna’s COVID-19 Vaccine Targeting SARS-CoV-2 Variant JN.1
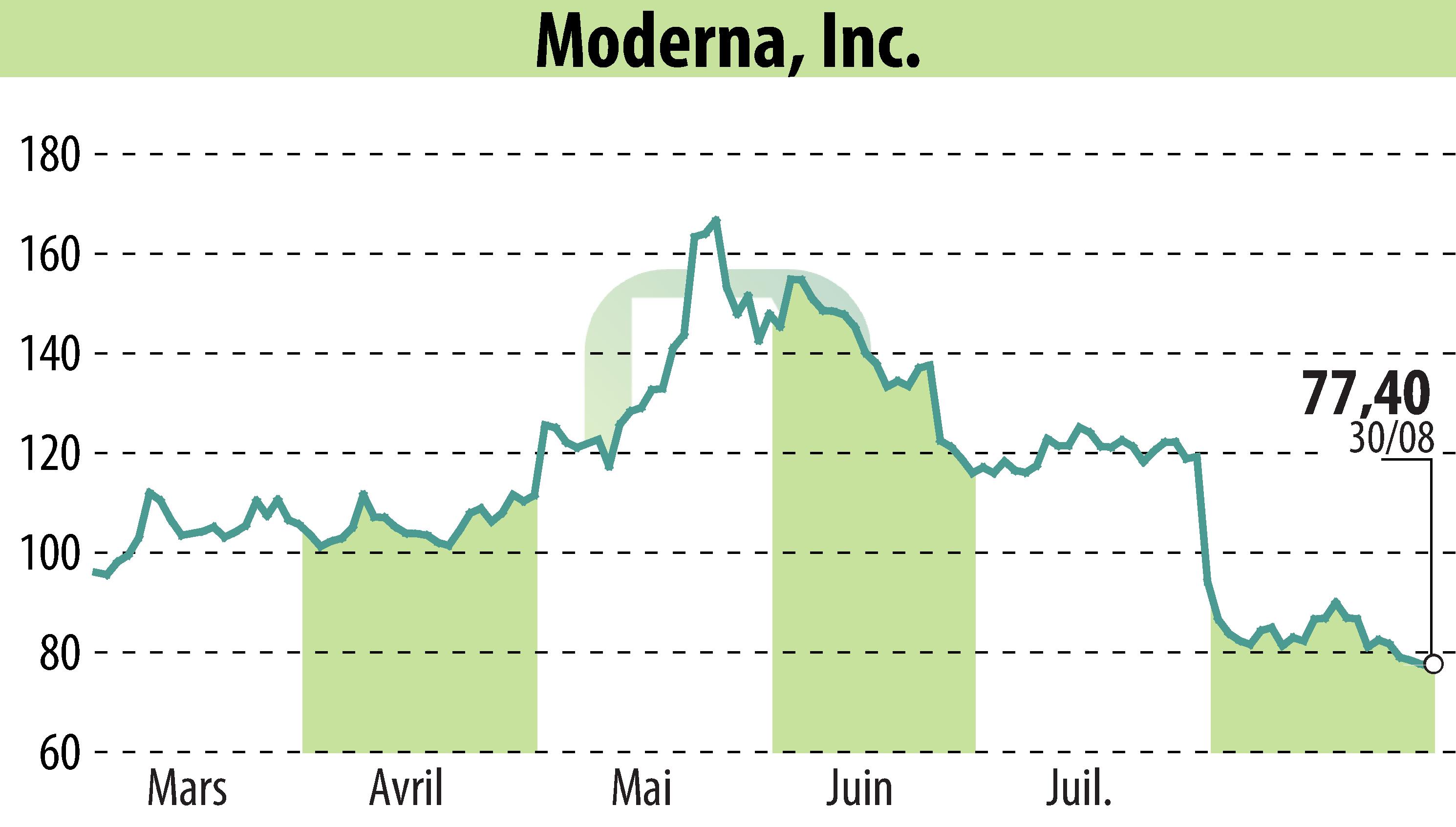
Cambridge, MA / ACCESSWIRE / September 2, 2024 / Moderna, Inc. (NASDAQ:MRNA) announced that the Taiwan Food & Drug Administration has approved an updated formulation of its COVID-19 mRNA vaccine, Spikevax. This new version targets the SARS-CoV-2 variant JN.1 and is authorized for active immunization in individuals aged six months and older.
In April 2024, the World Health Organization’s Technical Advisory Group on COVID-19 Vaccine Composition recommended the use of a monovalent JN.1 lineage for COVID-19 vaccine composition. Regulatory applications for Moderna's updated vaccine are currently under review worldwide, with decisions expected soon.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
