on Tharimmune Inc. (NASDAQ:THAR)
Tharimmune Secures EMA Feedback for TH104 in Chronic Pruritus
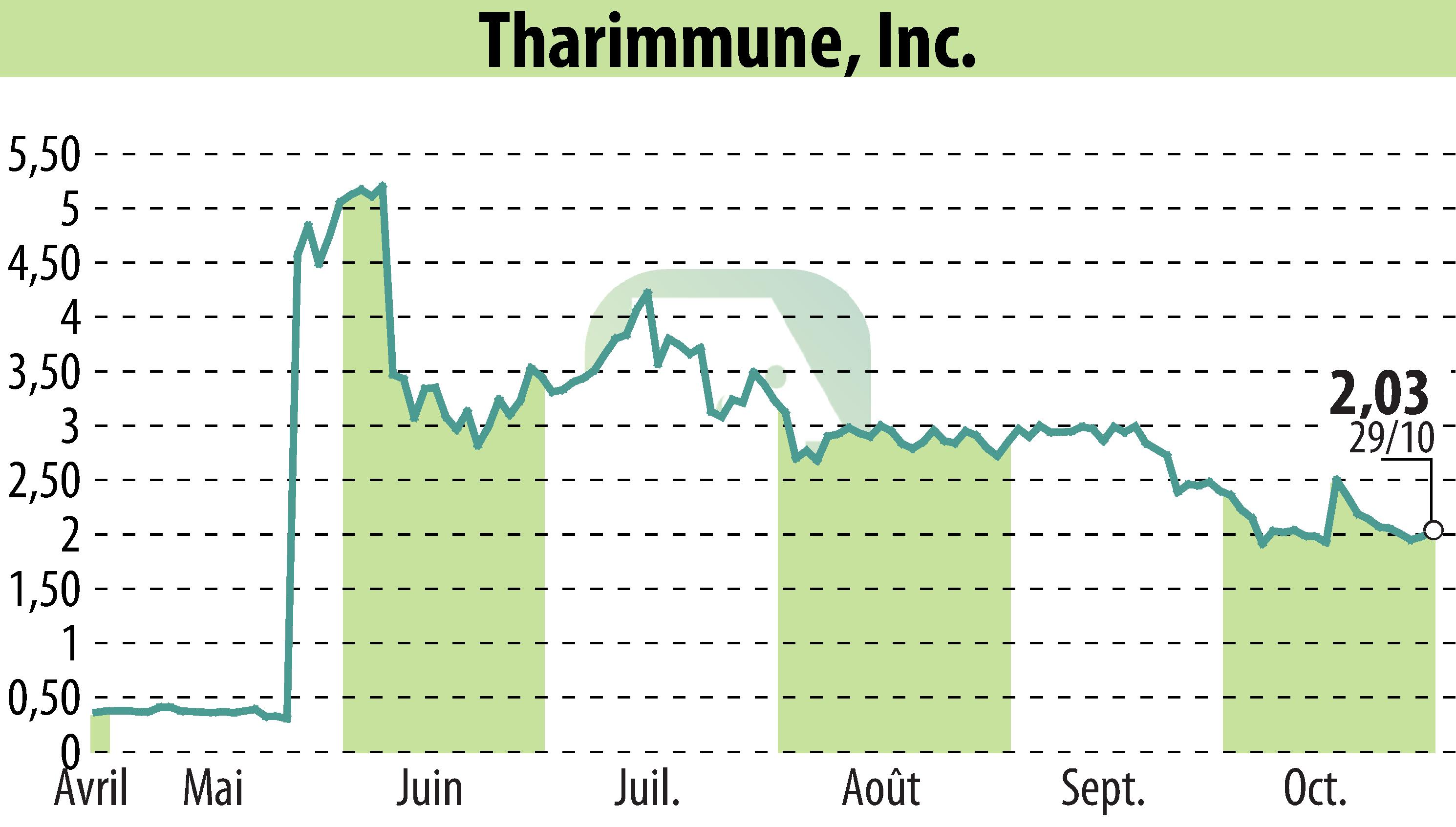
Tharimmune, Inc., a biotechnology firm focusing on inflammation and immunology, has received positive regulatory feedback from the European Medicines Agency (EMA) for its TH104 clinical program. This feedback specifically pertains to both the Phase 2 and Phase 3 trials for treating moderate-to-severe pruritus in primary biliary cholangitis (PBC). The EMA supports using a hybrid application approach, referencing existing non-clinical and some safety data, reducing the need for additional animal studies.
The agency endorsed the Phase 2 trial design, with some comments, and provided guidance for a future Phase 3 study. Tharimmune aims to begin a Phase 2 multiple-ascending dose trial soon, focusing on TH104’s safety and efficacy in PBC-related pruritus. CEO Randy Milby highlighted the importance of this guidance, which aligns with feedback from the FDA.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Tharimmune Inc. news
