on VALBIOTIS (EPA:ALVAL)
Valbiotis Announces Success in HEART II Study on Lipidrive
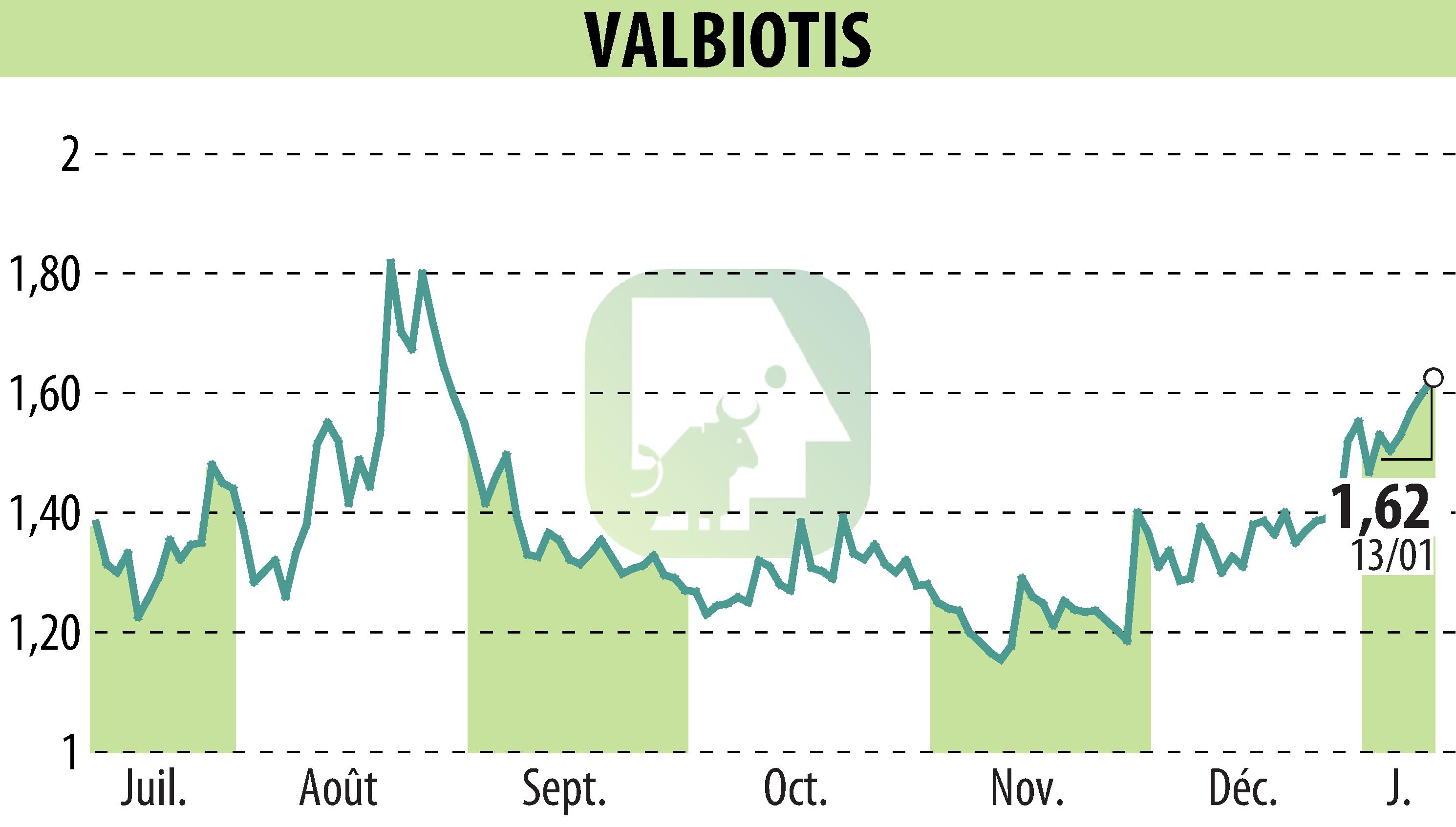
Valbiotis SA has announced a significant achievement in their phase II/III HEART II clinical trial concerning Lipidrive, the active ingredient in Valbiotis Pro Cholesterol. This study focused on individuals with mild to moderate hypercholesterolemia and demonstrated a marked reduction in LDL cholesterol, surpassing expectations compared to placebo results. The success confirms Lipidrive's safety and tolerability, with compliance rates exceeding 98%.
The clinical development included over 300 patients and validated the efficacy and safety of Lipidrive. Conducted across multiple centers, the international, placebo-controlled study included 180 subjects. Valbiotis plans to present the complete findings at upcoming international conferences and submit them for publication in peer-reviewed journals.
Sébastien Peltier, Valbiotis CEO, expressed pride in the results as they align with the company's goal to provide natural, clinically-proven health solutions, enhancing public health by addressing metabolic and cardiovascular issues.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALBIOTIS news
