on VALNEVA (EPA:VLA)
Valneva Achieves UK Approval for World's First Chikungunya Vaccine
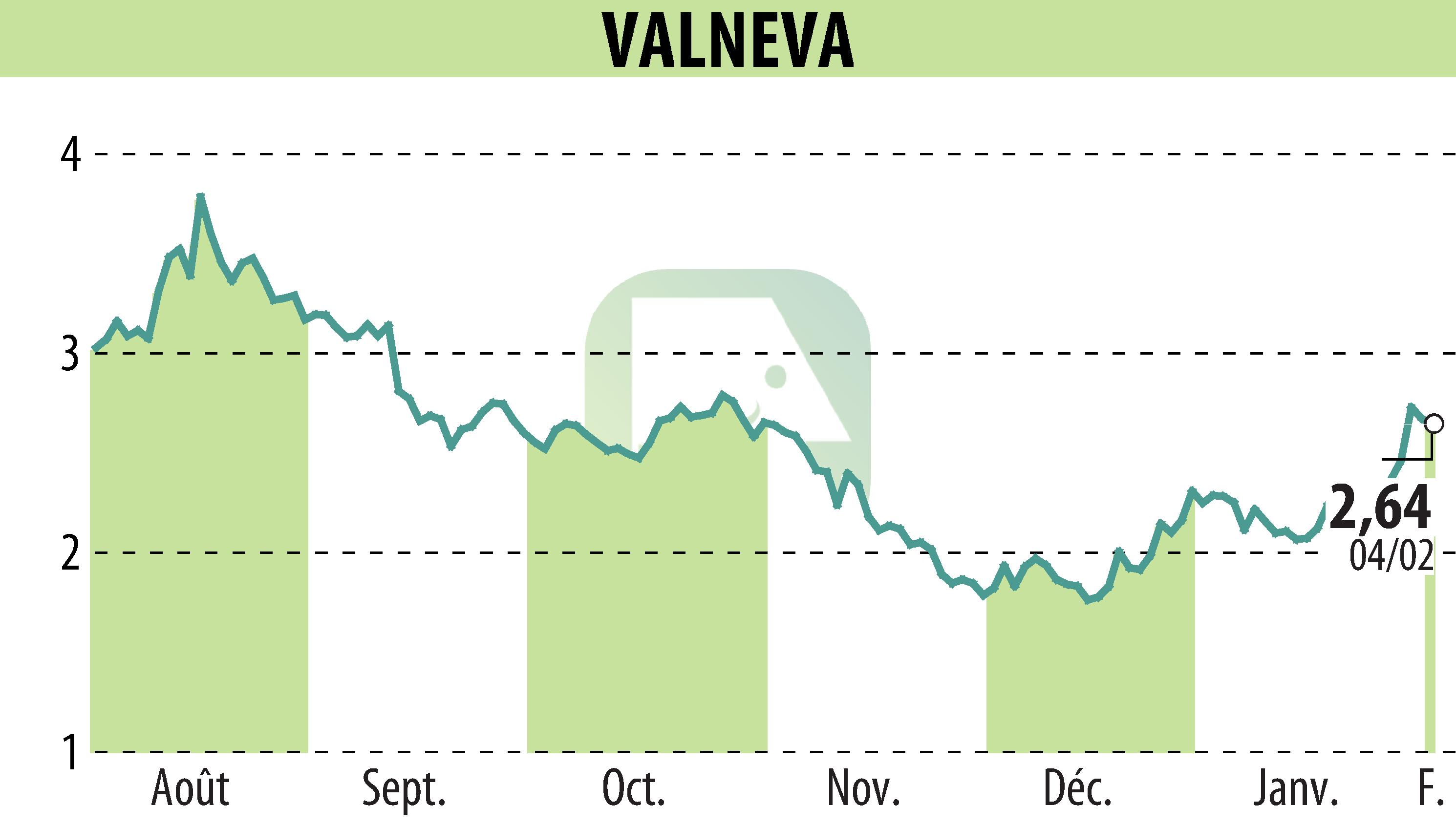
Valneva SE announced that the UK Medicines and Healthcare products Regulatory Agency has approved their chikungunya vaccine, IXCHIQ®, for individuals 18 and older. This marks the vaccine's fourth regulatory approval following prior authorizations in the United States, Europe, and Canada. Manufactured in Scotland, the single-dose vaccine showed a strong immune response in clinical trials. Valneva plans to seek further label expansions, targeting younger age groups.
The vaccine's approval coincides with rising chikungunya cases, particularly in India, a popular destination for UK travelers. This approval addresses both traveler safety and public health concerns over potential disease transmission upon return to the UK.
The decision aligns with global efforts to increase vaccine accessibility in endemic regions. Partnerships, including those with CEPI and the Serum Institute of India, aim to provide affordable vaccine options for low and middle-income countries.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
