on VALNEVA (EPA:VLA)
Valneva's IXCHIQ® Vaccine Authorized in Canada for Ages 12 and Older
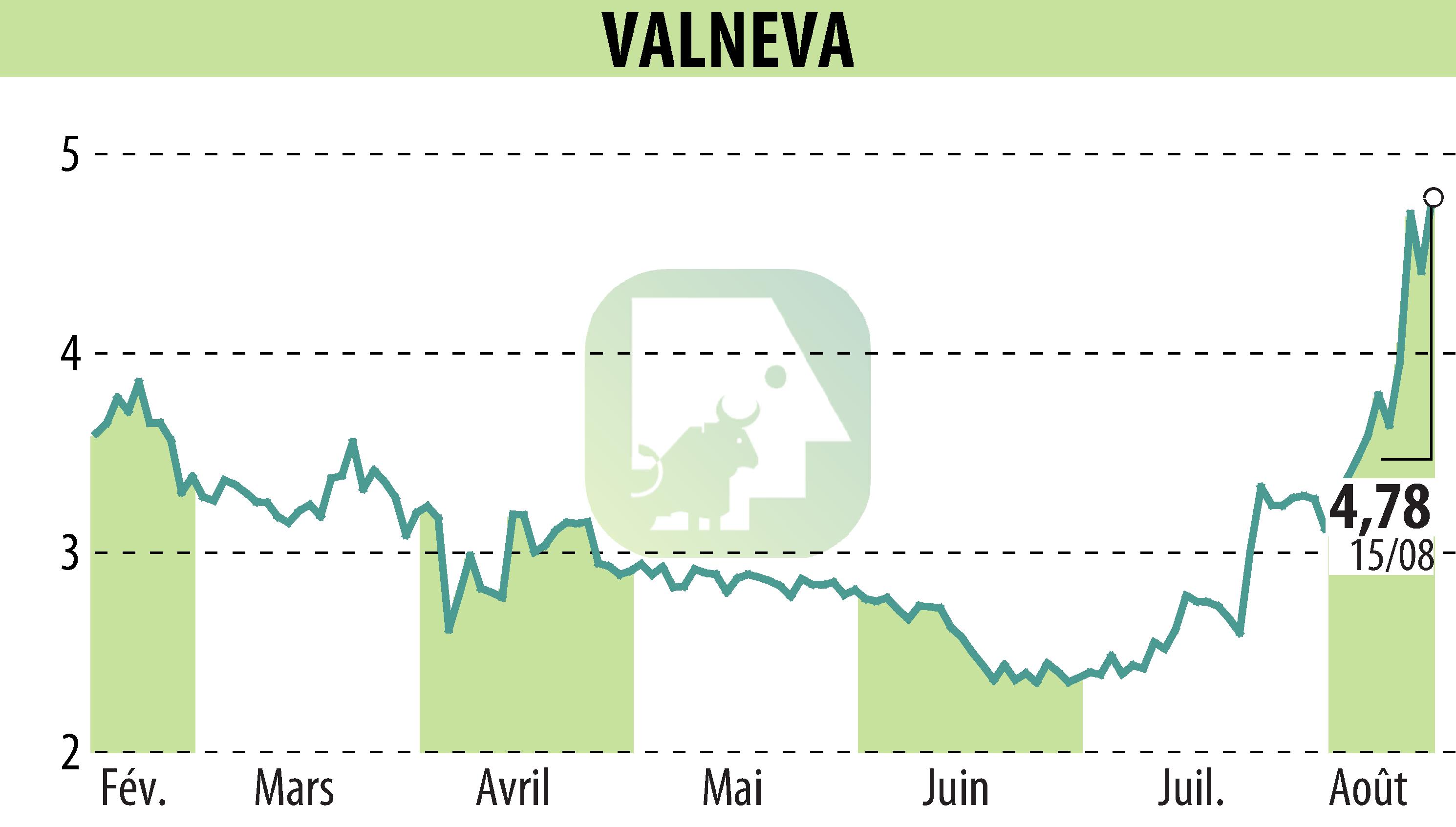
Valneva SE announced Health Canada's authorization of its chikungunya vaccine, IXCHIQ®, for individuals 12 years of age and older. This decision expands the initial authorization granted to adults, similar to the extension obtained in Europe for adolescents. Data submitted to Health Canada demonstrate prolonged antibody persistence over 24 months in 97% of participants.
Dr. Juan Carlos Jaramillo emphasized the importance of covering all age groups, as chikungunya spreads to previously unaffected areas. Through a partnership with CEPI, Valneva hopes to expand access to its vaccine in low- and middle-income countries.
As a reminder, IXCHIQ® was the first chikungunya vaccine approved in Brazil. This approval in Canada could facilitate other global approvals.
R. P.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
