on VALNEVA (EPA:VLA)
Valneva's Lyme Disease Vaccine Makes Significant Progress
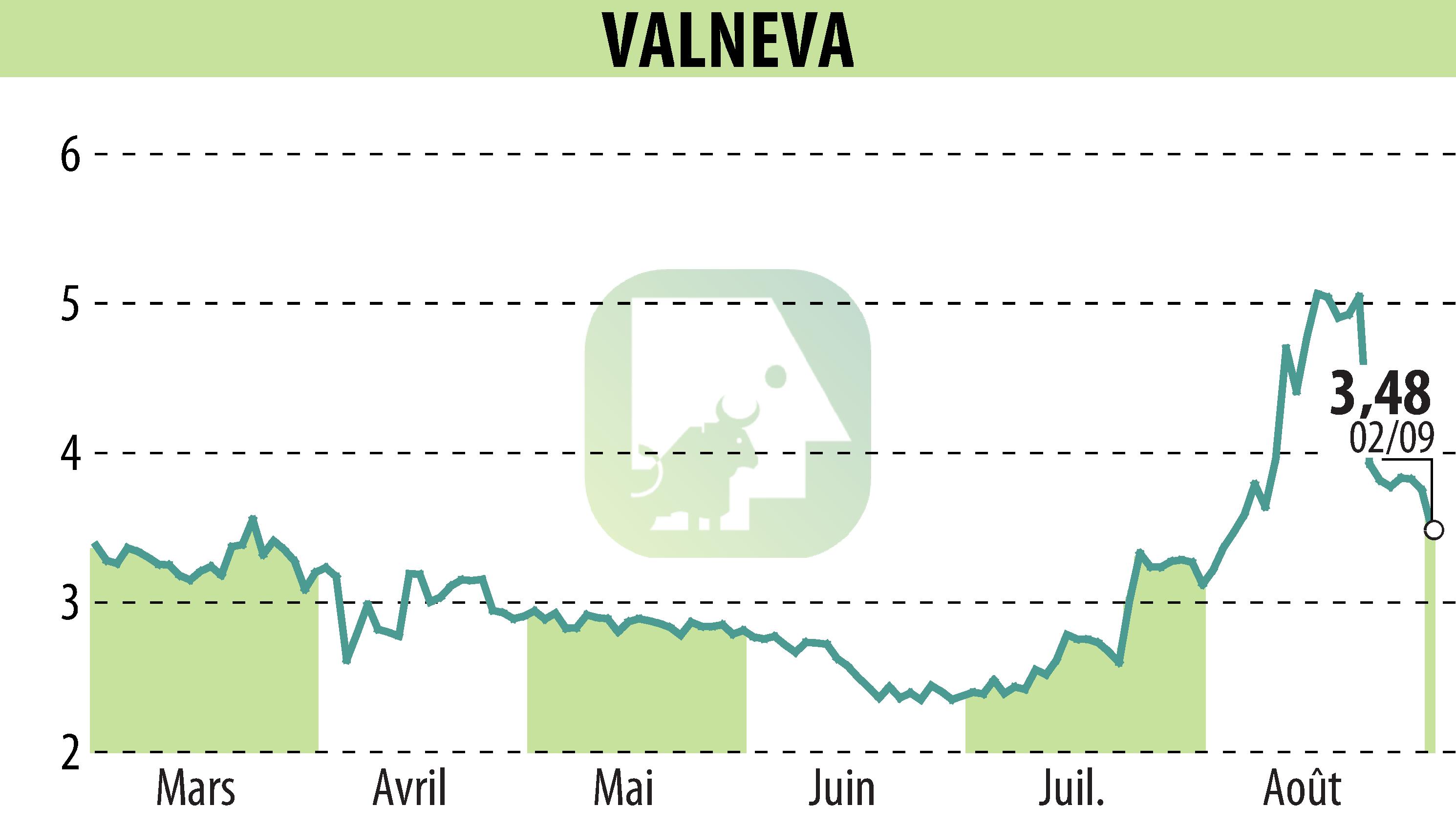
On September 3, 2025, Valneva SE announced promising Phase 2 results for its Lyme disease vaccine candidate, VLA15. The data show a strong immune response after a third booster dose in children and adults, with a notable anamnestic response for all six targeted serotypes.
No safety issues have been reported, corroborating previous findings. Currently, there is no human vaccine against this disease, which is spreading rapidly. The two ongoing Phase 3 trials could lead Pfizer to submit marketing authorization applications in 2026.
The Phase 3 study is examining the efficacy and safety of VLA15, which is being administered in endemic areas of North America and Europe. The development of this vaccine, in collaboration with Pfizer, addresses a growing medical need.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
