on VALNEVA (EPA:VLA)
Valneva Seeks FDA Approval for Chikungunya Vaccine Expansion
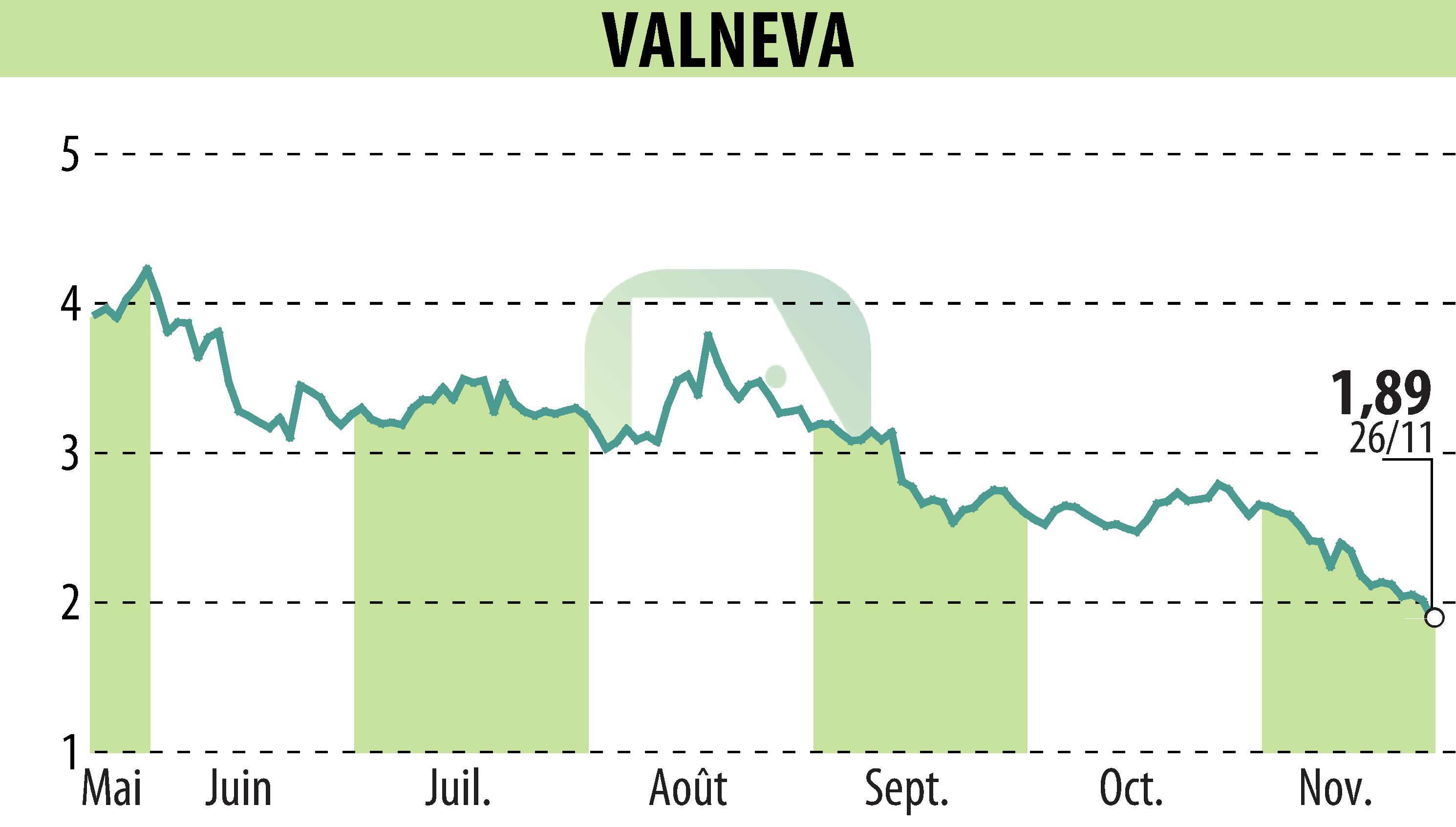
Valneva SE has submitted an application to the U.S. FDA for extending the use of its chikungunya vaccine, IXCHIQ®, to adolescents aged 12 to 17. Currently approved for adults, this application aims to include data on two-year antibody persistence as well. The move follows earlier submissions to the EMA and Health Canada based on positive Phase 3 results showing 99.1% immune response sustainability in adolescents. Similarly, the vaccine demonstrated a well-tolerated profile in this age group, substantiated by a study in The Lancet Infectious Diseases.
Alongside adolescent data, the long-term data indicate 97% persistence of the immune response after two years, proving equally durable across all ages. Valneva's Chief Medical Officer emphasizes the vaccine's importance for endemic regions where access can be challenging. Currently, IXCHIQ® is the first licensed chikungunya vaccine and is approved in the U.S., Europe, and Canada. Valneva plans to secure more global approvals and expand access through collaborations like that with CEPI.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
