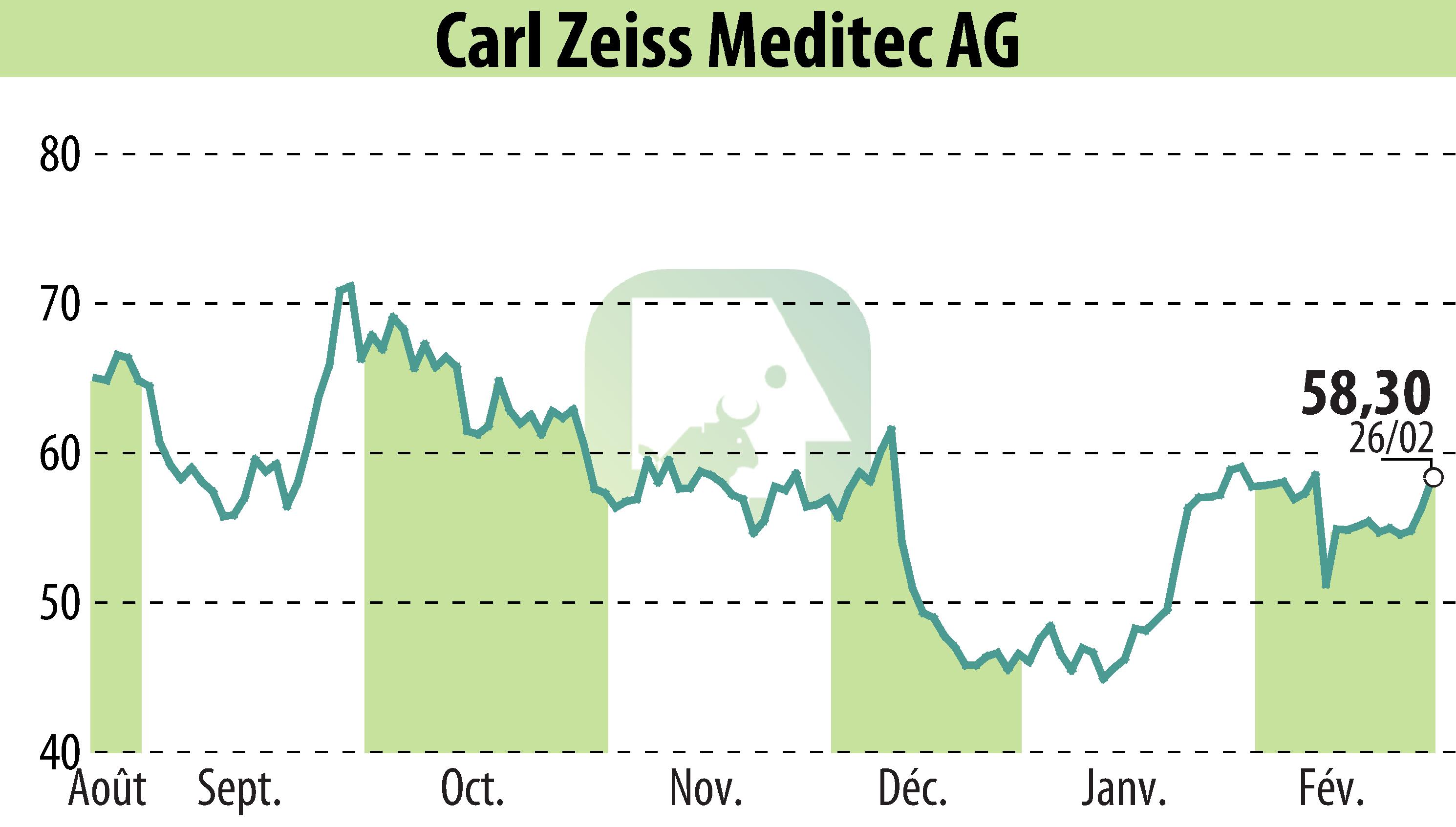
on Carl Zeiss Meditec AG (isin : DE0005313704)
ZEISS VISUMAX 800 Gains China Approval
ZEISS Medical Technology has obtained approval from China's National Medical Products Administration for its VISUMAX® 800 with SMILE® pro software. This marks the entry of ZEISS's latest femtosecond laser into China's market, aiming to improve laser vision correction procedures for nearsightedness, with or without astigmatism. The technology benefits from global success, demonstrated by over 10 million surgeries performed worldwide.
The VISUMAX® 800 offers faster treatments due to a laser pulse rate of 2 MHz. This reduces procedure time, which in turn lessens stress for both patients and surgeons. Additionally, its compact design ensures compatibility with various clinical environments. Workflow is enhanced through features such as CentraLign® for easier centration and OcuLign® to counteract cyclotorsion.
ZEISS emphasizes providing advanced solutions for refractive surgeons in China, highlighted by data-driven capabilities that optimize surgical paths and efficiency. This approval represents significant market potential for laser vision correction advancements across China.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Carl Zeiss Meditec AG news
