from Methapharm
Methapharm Announces Partnership With Bosch
Distribution of the Newest FDA-Cleared FeNO Device, Vivatmo Pro, in the United States
CORAL SPRINGS, FL / ACCESSWIRE / May 21, 2024 / Today, Methapharm Respiratory, a global leader in respiratory diagnostics, announced a new partnership with Bosch Healthcare Solutions, a wholly owned subsidiary of the Robert Bosch GmbH. The Bosch Group is a leading supplier of technology and services, with roughly 429,000 associates worldwide.
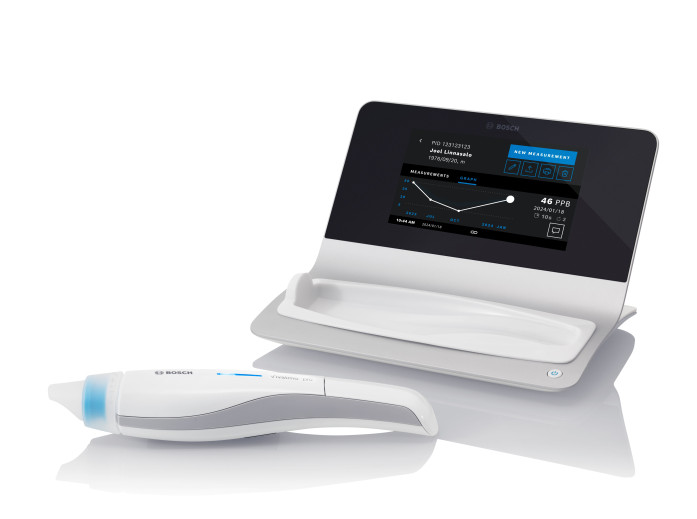
Vivatmo pro - Base station with handheld next to it
"Methapharm Respiratory has gained a global reputation as a trusted provider in respiratory diagnostics," said Craig Baxter, CEO of Methapharm. "Partnering with Bosch will expand our portfolio with an outstanding diagnostic product, bolstering our vision, mission, and position in the industry. Bosch's high level of technological expertise built over decades and its dedication to innovate is in line with Methapharm's commitment to meet the needs of our clinical partners and patients. We are excited to partner with Bosch, a trusted technology brand striving to improve the quality of life."
The FDA has cleared the sale of Vivatmo pro from Bosch in the United States.
More information about Vivatmo pro for the U.S. can be found at
https://methapharmrespiratory.com/vivatmopro
Vivatmo pro is a medical device intended for use by qualified healthcare professionals to perform point-of-care measurements, primarily on asthma patients. Vivatmo pro measures fractional exhaled nitric oxide in human breath (FeNO). FeNO is an increasingly important marker for airway inflammation that is used in asthma management1 to support the diagnoses and individualized therapy. Providing physicians with an enhanced ability to assess airway inflammation can help ensure that patients receive the medication and treatment they need 2. The design award-winning Vivatmo pro device is non-invasive, easy to use, and virtually maintenance-free utilizing high-precision Bosch sensor technology.
"Methapharm is the ideal partner for our entry into the U.S. medtech market," states Marc Meier, General Manager of Bosch Healthcare Solutions. "We work with great passion and engineering skills to make everyday life in the healthcare sector better, step by step. Methapharm's educational and dialog-based approach ensures that we have access to our U.S. customers at eye level and understand their needs. This helps us to tailor our high-precision sensor technology and the resulting asthma management solutions to the requirements of the market. This fits our company philosophy perfectly: Invented for Life."
About Methapharm Respiratory
Methapharm Respiratory specializes in the promotion and distribution of diagnostic products that enhance respiratory care. Proprietary products are marketed in North America and globally through partnerships with distributors. Methapharm Respiratory is a division of Methapharm Specialty Pharmaceuticals, a privately held company with a focus on specialty pharmaceutical products. The company began operating in 1996 and partners with a number of leading international pharmaceutical companies to market and distribute specialty products covering a wide range of therapeutic areas in North America.
About Bosch Healthcare Solutions
Bosch Healthcare Solutions GmbH is a wholly owned subsidiary of Robert Bosch GmbH. The subsidiary was established in 2015 with the aim of developing products and services that improve people's health and quality of life. Almost 300 associates (2024) are currently employed at the company's headquarters in Waiblingen, Germany. The subsidiary's solutions draw on the Bosch Group's core competencies: highly precise, miniaturized sensors to collect data, software to evaluate data, and services based on data analysis. For more information about Bosch Healthcare Solution, please visit bosch-healthcare.com.
1 Neveda Murugesan et al. 2023. Update on the Role of FeNO in Asthma Management. https://doi.org/10.3390/diagnostics13081428
2 Truong-Thanh T et al. 2020. The beneficial role of FeNO in association with GINA guidelines for titration of inhaled corticosteroids in adult asthma: A randomized study. https://doi.org/10.1016/j.advms.2020.03.001
Contact Information
Matthew Baxter
mbaxter@methapharm.com
Sherian Kington
skington@methapharm.com
SOURCE: Methapharm, Inc.
View the original press release on newswire.com.

